
Our Laboratory
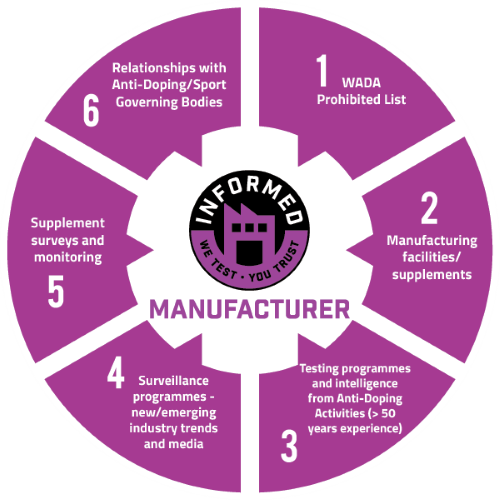
LGC, the globally recognised testing laboratory behind Informed Manufacturer, is a world-renowned sports doping control and research laboratory, with over 60 years of experience of regulatory analysis. With over 400 published scientific papers and over 500 person-years of research into doping control, LGC is recognised as a global leader and trusted partner in the field of anti-doping and dietary supplement analysis.
LGC has been testing supplements, ingredients and manufacturing sites for more than 15 years. During this time, more than 225,000 samples have been tested and a number of research studies have been conducted; such as studies exploring the prevalence of prohibited substance contamination within supplement products.
To understand the risks elite athletes face, LGC conducts administration studies which have been published in peer-reviewed scientific journals. These studies have highlighted that consuming even microgram quantities of a prohibited substance (millionths of a gram) could give rise to a positive doping violation. LGC has also published white papers highlighting research on supplement contamination.
The field of anti-doping is continually changing, with new and emerging threats/risks continuing to challenge the supplement industry and the anti-doping community. To address these challenges, LGC is continuously developing and evolving screening methodology, with ongoing method development and research programmes conducted by our specialist laboratory. To provide assurance to brands and consumers alike, LGC routinely screens supplement products for in excess of 285 banned in sport and/or compound considered harmful to health. This includes drugs of abuse, anabolic agents, stimulants, beta-2 agonists, diuretics and new and emerging threats.
As a doping control laboratory, LGC works directly with sports authorities, national anti-doping organisations and national governing bodies worldwide. Providing advice and expert support, LGC is able to represent the views of partners and assist in the shaping of future regulation.
Testing Specifications
LGC has shaped its screening programme for Informed Manufacturer by conducting surveillance analysis and research in order to assess manufacturing supplement contamination and the emergence of new threats within manufacturing testing facilities. LGC works closely with a number of key stakeholders within the anti-doping community and supplement industry, to ensure that quality certification for manufacturing sites remains at the forefront of risk management.
Download Our Detection of Synthetic Pharmaceutical Compounds White Paper
LGC currently tests over 25,000 samples each year for in excess of 285 substances which are prohibited in sport and/or considered a risk in relation to supplement contamination. Screening includes, but is not limited to, testing for compounds such as anabolic agents, stimulants, narcotics, beta-2 agonists, diuretics and new and evolving threats such as vaptans and PPAR's etc.
|
LGC is continuously reviewing and developing its testing methodology to ensure new and emerging threats within the manufacturing supplements industry are addressed - this often includes the surveillance and investigation of suspicious compounds before their prohibited status is confirmed by the wider anti-doping community.






